Part 1 of 2. 8-minute read. // (Photo: The cholesterol particles circulating in blood vessels come in a variety of forms. CREDIT: DANIEL GARCIA)
When C. Michael Gibson of Boston saw his doctor in the spring of 2023, the blood test results were confusing. His cholesterol levels were decent — he was already taking statins to keep the “bad” cholesterol low — but the arteries delivering blood to his heart were nonetheless crammed with dangerous plaque. “It didn’t make sense,” says Gibson, himself a cardiologist at Beth Israel Deaconess Medical Center.
So Gibson asked his physician to check his blood for a specific kind of cholesterol called lipoprotein(a). And there was the explanation: He had more than double the normal amount of that cholesterol. Gibson turned out to be one of the unlucky people who has inherited a predisposition toward high lipoprotein(a) levels; he suspects that his grandfather, who died of a heart attack at age 45, had it too.
About one in five people have this unfortunate heritage, and there’s nothing they can do to combat it — but soon that might change. Scientists are researching medications that can lower lipoprotein(a), as well as other approaches that could slash the risk of cardiovascular disease more than drugs like statins can.
Statins, approved in the late 1980s to lower levels of low-density lipoprotein (LDL) cholesterol, have been a lifesaving tool: They cut risk of heart attack and stroke by up to 50 percent for the more than 200 million people globally who take the medications. Yet even statin takers still get heart disease, and some still die. Cardiovascular disease remains the leading cause of death in the United States and across the world. Clearly, something’s been missing from the cholesterol picture.
The picture coming into focus today incorporates not just bad, LDL cholesterol and good, high-density lipoprotein (HDL) cholesterol, but also lipoprotein(a) and a poorly understood substance called “remnant cholesterol.” Medical researchers aim to minimize all of these except HDL. And HDL cholesterol itself, though it’s still understood to be beneficial, has turned out to be more complex than anticipated. Various attempts to raise HDL levels haven’t improved people’s health beyond what statins already achieve.
Yet despite this and other disappointments in which medicines haven’t panned out as expected, many researchers feel optimistic about treatments currently in clinical trials. “It’s really an exciting time,” says Stephen Nicholls, a cardiologist at Monash Health in Melbourne, Australia.
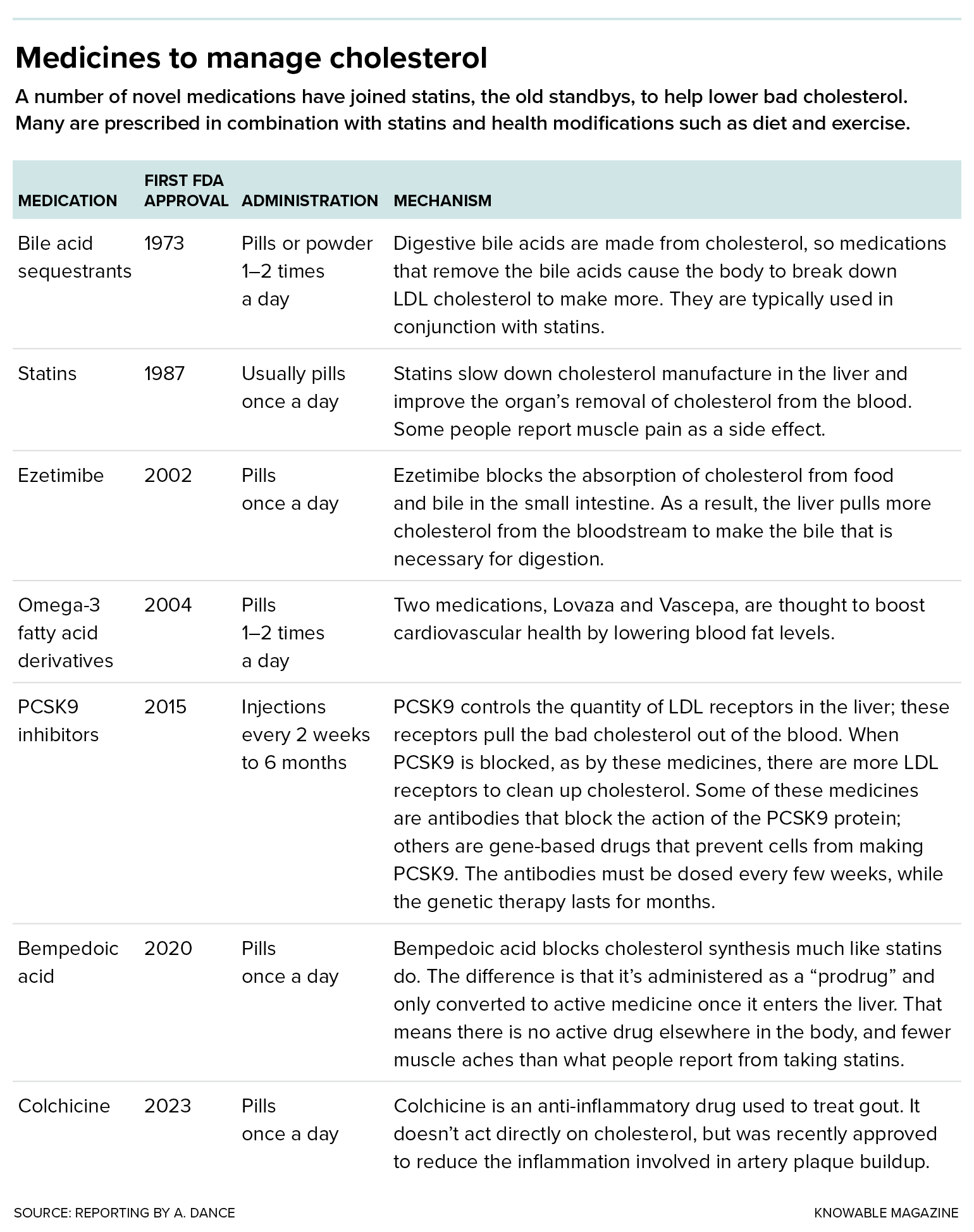
The list of drugs used to address high LDL cholesterol is growing.
LDL cholesterol
Though it gets a bad rap among the health-conscious, cholesterol plays important roles in our body: It helps to control the stability and fluidity of cell membranes and is an important starting ingredient for making hormones such as testosterone and estrogen. What matters for our health is the company that the cholesterol molecule keeps when it travels.
Its waxy nature means it can’t mix well with water, so it can’t pass through the bloodstream on its own: Lone cholesterol molecules would separate out, like oil does in water. Cholesterol’s solution is to join up with complexes of proteins and fats, called lipoproteins, that carry it around. These lipoprotein carriers include LDL, HDL and other types. Cholesterol, in addition to being cargo, is a structural part of these carriers, too.
Lipoproteins are made in the gut and liver, and they deliver cholesterol and fat to body tissues. Fat goes to muscles, to be used for energy, or to fat tissue for storage. Cholesterol is dropped off in tissues to be incorporated into cell membranes or made into hormones. Cholesterol can also be returned to the liver where it can be stored, incorporated into new lipoproteins, turned into bile acids used by the digestive system to break down fats, or sent to be excreted.
When the delivery particles from the liver have dropped off most of their fats, they become LDL particles, which are still jam-packed with cholesterol. The problem happens when these LDL particles, instead of returning to the liver to be recycled, squeeze into blood vessel walls and get chemically modified. There, they incite or exacerbate an immune reaction called inflammation. In response, immune cells come in to eat LDL particles — but if they eat too much, they can get stuck in the blood vessel wall. This forms the beginnings of an atherosclerotic plaque.
Over time, that plaque accumulates more cholesterol, more fat and more immune cells, reducing the space through which blood can flow and deliver oxygen to tissues. If a plaque limits blood supply to the heart, it might cause chest pain called angina. A plaque might also lead to formation of a blood clot, which may break off and clog vessels elsewhere. The clot might cause a stroke in the brain, for example, or a heart attack.
Today, it’s clear that the less LDL cholesterol in the bloodstream, the better. Statins are good at achieving this, cutting LDL cholesterol levels by up to about half. And for those who need a bigger effect, or who can’t tolerate statins (muscle pain or weakness is an occasional side effect), there are newer medicines. “We now have the ability to get almost anyone’s LDL cholesterol down into the range that we would consider appropriate,” says Steven Nissen, a cardiologist at the Cleveland Clinic in Ohio.
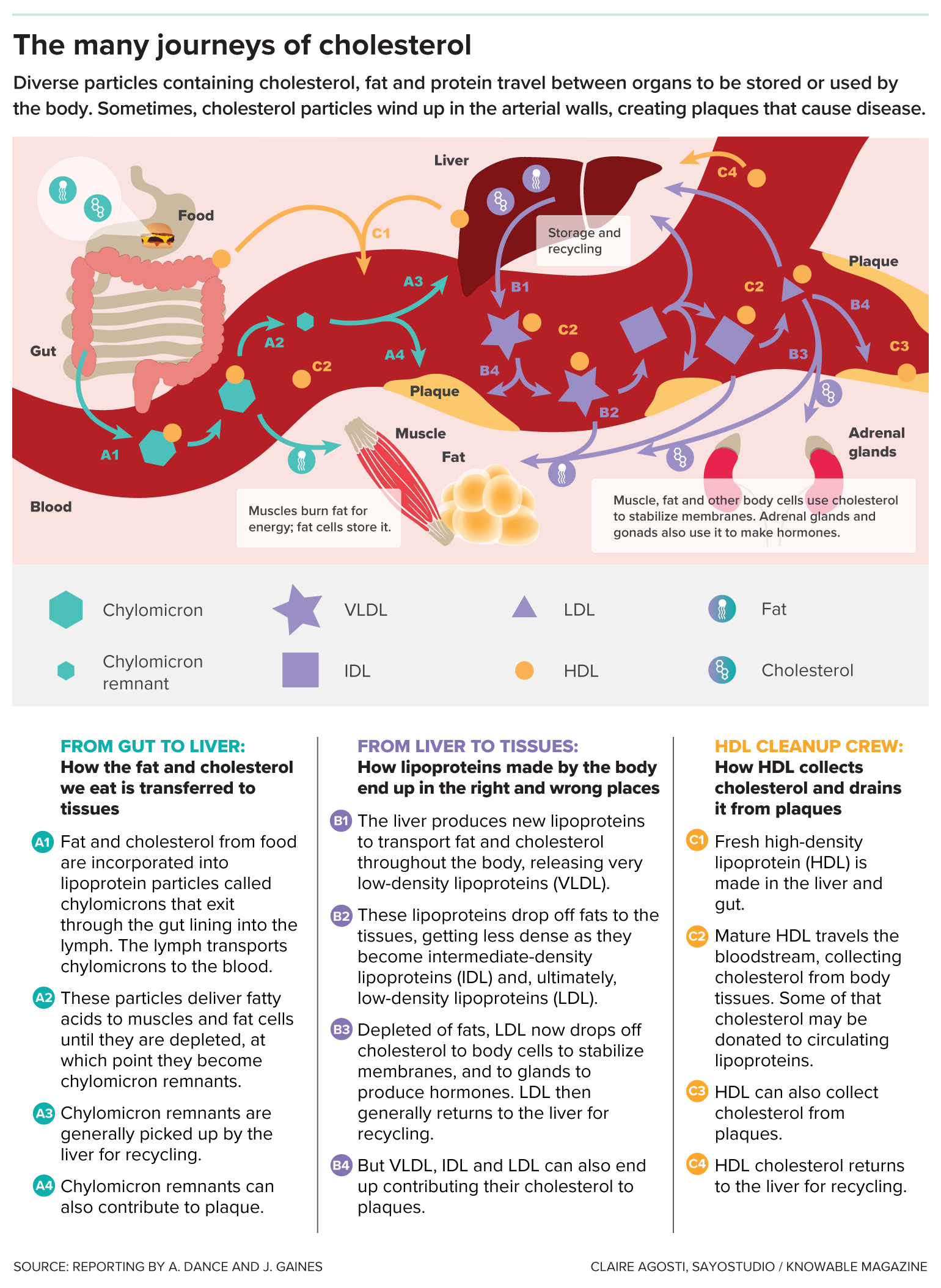
As cholesterol moves around the body within various protein- and fat-containing particles, it aids in important functions but also has the potential to create health risks.
Lipoprotein(a)
But these LDL-cholesterol treatments generally don’t do much against levels of lipoprotein(a), pronounced “lipoprotein-little-a.” This substance, composed of LDL cholesterol particles plus an extra protein, apolipoprotein(a), is mysterious: Scientists don’t know what its natural job is, though since apolipoprotein(a) has some similarity to a protein involved in blood clotting, it might have a role in wound healing. But it can’t be all that important to animal survival: Weirdly, the gene that carries instructions for making apolipoprotein(a) is found only in certain primates. (A similar gene evolved in hedgehogs.)
It’s also unclear why lipoprotein(a) is such a bad version of cholesterol, but it’s clearly up to no good much of the time. It delivers cholesterol to the blood vessel walls like LDL does, promotes blood clotting that blocks arteries and can cause inflammation and increase the risk of clots. And if your lipoprotein(a) is high — too bad. “Statins won’t get it down,” laments Gibson. “Exercise doesn’t get it down. Diet doesn’t get it down.”
Some of the newer LDL cholesterol-lowering drugs can reduce lipoprotein(a) cholesterol a bit, but probably not enough to significantly reduce cardiovascular risk, says Anand Rohatgi, a cardiologist at the University of Texas Southwestern Medical Center in Dallas. The only thing physicians can do, in extreme cases, is to regularly administer a blood-cleaning procedure called apheresis to remove lipoprotein(a).
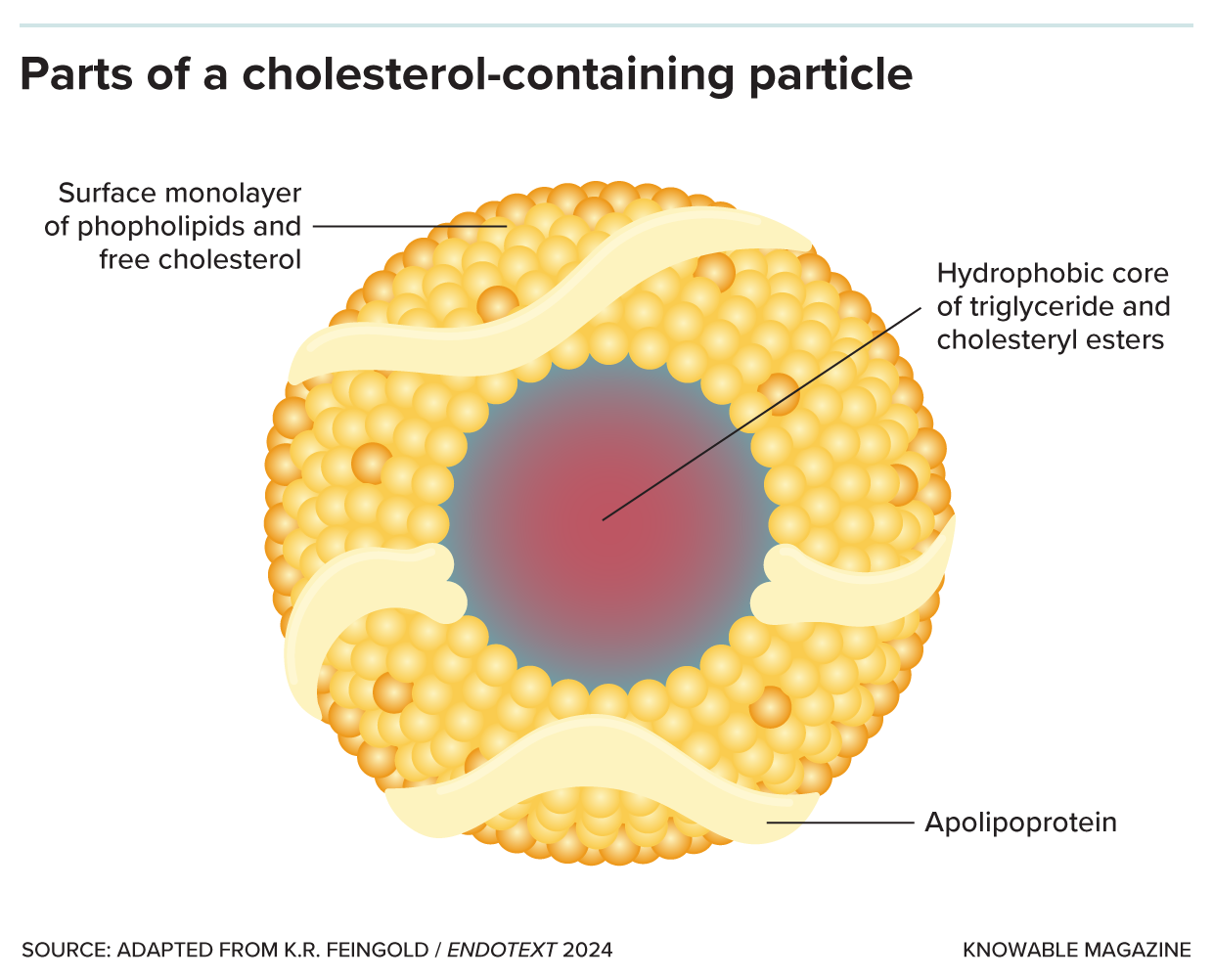
Lipoprotein particles are made up of a core containing fat in the form of triglycerides and cholesterol in the form of cholesteryl esters, surrounded by phospholipids, free cholesterol molecules and apolipoprotein.
For a long time, doctors ignored lipoprotein(a). “Nobody measured it, because you could not do anything about it,” says Prakriti Gaba, a cardiologist at Brigham and Women’s Hospital in Boston. That may be about to change now that several groups are testing medicines that target the substance. (Gaba got her own levels checked at a cardiology conference, where booths offering free tests have sprung up recently.)
Many of these experimental medications use genetic technology to silence the apolipoprotein(a) gene. In a handful of small studies, involving dozens to a few hundred subjects each, different apolipoprotein(a)-silencing therapies cut lipoprotein(a) levels by varying levels, from no change up to 92 percent. But it isn’t yet known whether cutting lipoprotein(a) will actually reduce cardiovascular problems. “We won’t know for a while,” says Leslie Cho, a cardiologist at the Cleveland Clinic who’s coleading one of the trials.
Cho’s HORIZON study, the farthest along, is testing a lipoprotein(a)-gene-silencing treatment compared to a placebo in more than 8,300 people with high lipoprotein(a) and a history of heart problems such as heart attack or stroke. The hope is that reducing lipoprotein(a) will decrease the rate of heart attacks, strokes, need for a medical procedure to improve blood flow, and death, but HORIZON isn’t expected to have results until 2025. Another trial that Gaba is involved in, called OCEAN(a)-Outcomes, is testing a similar approach in about 6,000 people, but is not expected to be completed until the end of 2026.
To be continued...











